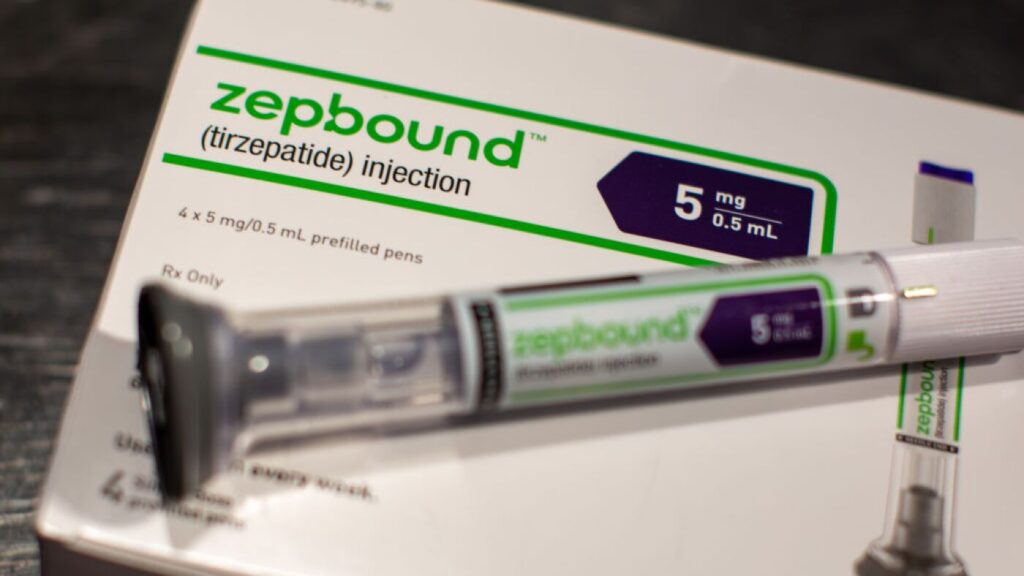
Eli Lilly & Co., the manufacturer of the weight loss drug Zepbound, is suing four telehealth companies for allegedly selling illegal copies of drugs made by compounding pharmacies. Compounding pharmacies can create custom medications during drug shortages, but Eli Lilly contends that the versions being sold are unauthorized and potentially harmful to patients. The active ingredient in Zepbound, tilzepatide, has been in shortage for two years, leading patients to seek more affordable compounded alternatives, which can be sold for about $99 compared to the branded drug’s price of over $1,086 per month.
With the shortage now over, Eli Lilly is taking action against companies like Mochi Health, Willow Health, Fella Health, and Henry Med for violating laws against copying existing drugs. Lilly’s complaints assert that these companies have used misleading practices and made unauthorized modifications to the formulations. For example, Mochi allegedly altered the composition of tilzepatide and the dosages given to patients. The lawsuits accuse these telehealth companies of making false claims about the safety and efficacy of their compounded versions compared to the FDA-approved drug.
The lawsuits underscore the tension between the need for customization in healthcare and the regulatory framework governing drug production. Industry representatives have highlighted the complexity in distinguishing between legal customization and illicit replication, suggesting that these legal battles may ultimately require judicial interpretation.
Source link